What is D-Dimer ?
The D-Dimer test measures the presence of D-Dimer, a protein fragment produced when a blood clot dissolves. Elevated D-Dimer levels indicate that there is significant clot formation and breakdown in the body, which can be a sign of serious health conditions, such as deep vein thrombosis (DVT), pulmonary embolism (PE), or disseminated intravascular coagulation (DIC). This test is often used to rule out clot-related disorders. Clinicians may order a D-Dimer test to: Diagnose or rule out blood clotting disorders: Elevated D-Dimer levels suggest active clot formation and breakdown, aiding in the diagnosis of conditions like DVT and PE. However, a normal D-Dimer level can help rule out these conditions in low-risk patients. Evaluate suspected pulmonary embolism: In patients with symptoms like sudden shortness of breath, chest pain, or rapid heartbeat, the D-Dimer test helps determine if a PE may be present, warranting further imaging tests. Monitor treatment for DVT and PE: For patients undergoing treatment for blood clots, D-Dimer levels can be monitored to assess the effectiveness of treatment and to check if clots are resolving. Aid in diagnosing disseminated intravascular coagulation (DIC): DIC is a rare but life-threatening condition where abnormal clotting occurs throughout the body. Elevated D-Dimer is a common marker in patients with DIC. Assess clotting risk in COVID-19 patients: During the COVID-19 pandemic, D-Dimer testing became a valuable tool to assess clotting risk, as severe COVID-19 cases were associated with an increased risk of clot formation.
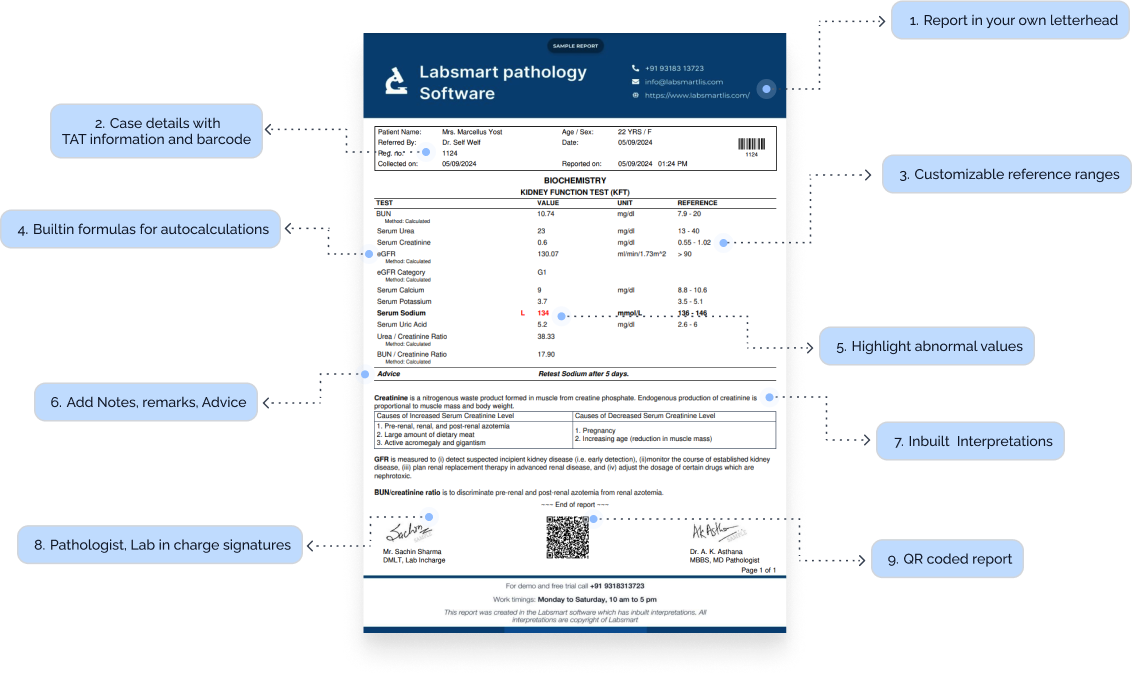
D-Dimer Report Format: Breakdown
Here’s what an ideal D-Dimer report format should include:
Header Information:
- Patient Details: Full name, Age, Gender, and ID.
- TAT information: Timestamp for both sample collection and report generation.
- Doctor's Information: Name of the referral doctor, if applicable.
Test Results Section:
-
Patient's results
As obvious as it is, a test report should definitely have the patient's test result.
-
Result's Unit
The unit of the test result must be mentioned correctly in the report. D-Dimer test is generally reported in “μg FEU/mL”
-
D-Dimer Normal Value / Reference Range
The report must have the normal D-Dimer range. It can differ slightly based on the reagents used by the lab and other internal factors, but common D-Dimer ranges are:
< 0.5 μg FEU/mL
Interpretations
Nowadays, most labs prefer to add interpretations to the reports, making the report more patient-friendly. Labsmart software has interpretations of all routine test pre-filled in the software.
Footer Section:
-
Certifications:
Display any relevant accreditations (e.g., NABL, ISO), adding to your lab's credibility.
-
Pathologist and technician signature:
It's mandatory to add a Pathologist and technician signature to the report.
D-Dimer Interpretation
In Labsmart software, this is the inbuilt interpretation for D-Dimer
Importance of adding interpretation to reports:
It's very helpful to add interpretation in reports as it makes the reports more patient friendly and also helpful to doctors in some cases. Moreover, presently most labs prefer providing reports with interpretation. Thus, adding interpretation to report will help your lab stay at par with other competitor labs.
Labsmart software
(With Interpretation and auto calculations)
- Billing
- Reports with Interpretation
- Auto calculation where needed
- Check daily business
- WhatsApp reports
* Free plan available

1.5 Crore+
Reports printed & delivered online
1250+
Labs Active
10+
Countries
D-Dimer MS Word format
Download the Ms word editable D-Dimer report format for offline reporting.
Download word format